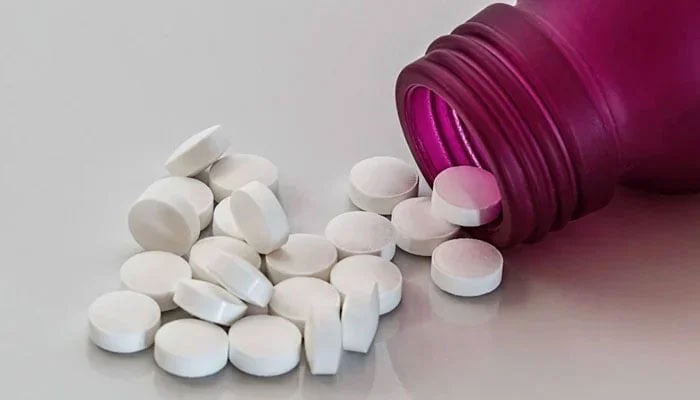
DRAP cancels all registrations of high-dose tramadol for export
DRAP has taken bolder step of cancelling all export approvals for 225mg and 250mg formulations outright
ISLAMABAD: In a major regulatory breakthrough, the Drug Regulatory Authority of Pakistan (DRAP) has cancelled all existing registrations of high-dose Tramadol formulations—specifically 225mg and 250mg variants—that were previously approved for export purposes.
The decision was taken during the 346th meeting of DRAP’s Registration Board as part of a broader move to align Pakistan’s pharmaceutical exports with international drug safety standards. The sweeping cancellation comes amid growing concerns over the misuse and trafficking of high-strength Tramadol, particularly in African and Middle Eastern countries, where these formulations are banned due to their high abuse potential.
The move has been widely praised by health professionals and regulatory observers as a strong signal of Pakistan’s commitment to global drug safety protocols. Under the leadership of its newly appointed CEO, Dr. Obaidullah, DRAP is being lauded for adopting the regulatory principles practiced by stringent authorities such as the U.S. FDA, UK’s MHRA, France’s ANSM, and Health Canada.
Officials in DRAP revealed that the decision to revoke registrations was influenced by discussions with Nigerian health authorities and representatives of the Global Rapid Interdiction of Dangerous Substances (GRIDS) program. These conversations identified Pakistan as a major source of high-strength Tramadol formulations—especially those exceeding 100mg—that were contributing to a public health crisis in several African nations. DRAP’s Controlled Drugs Division subsequently recommended a complete withdrawal of any Tramadol formulations not approved by international reference regulators. The board accepted the recommendations and initiated legal action under Section 42 of the Drugs Act, 1976. Show-cause notices were initially issued to 59 firms marketing 225mg and 200mg Tramadol formulations, with many of them called for personal hearings to justify why their registrations should not be cancelled. However, DRAP has now taken the bolder step of cancelling all export approvals for 225mg and 250mg formulations outright. Tramadol, a synthetic opioid used to treat moderate to severe pain, has been widely misused due to its addictive properties.
-
 Inside Kate Middleton, Prince William’s Nightmare Facing Andrew Mountbatten-Windsor
Inside Kate Middleton, Prince William’s Nightmare Facing Andrew Mountbatten-Windsor -
 Margaret Qualley Shares Heartfelt Confession About Husband Jack Antonoff: 'My Person'
Margaret Qualley Shares Heartfelt Confession About Husband Jack Antonoff: 'My Person' -
 Savannah Guthrie Shares Sweet Childhood Video With Missing Mom Nancy: Watch
Savannah Guthrie Shares Sweet Childhood Video With Missing Mom Nancy: Watch -
 Over $1.5 Million Raised To Support Van Der Beek's Family
Over $1.5 Million Raised To Support Van Der Beek's Family -
 Diana Once Used Salad Dressing As A Weapon Against Charles: Inside Their Fight From A Staffers Eyes
Diana Once Used Salad Dressing As A Weapon Against Charles: Inside Their Fight From A Staffers Eyes -
 Paul Anthony Kelly Opens Up On 'nervousness' Of Playing JFK Jr.
Paul Anthony Kelly Opens Up On 'nervousness' Of Playing JFK Jr. -
 Video Of Brad Pitt, Tom Cruise 'fighting' Over Epstein Shocks Hollywood Fans
Video Of Brad Pitt, Tom Cruise 'fighting' Over Epstein Shocks Hollywood Fans -
 Jelly Roll's Wife Bunnie Xo Talks About His Huge Weight Loss
Jelly Roll's Wife Bunnie Xo Talks About His Huge Weight Loss -
 Margot Robbie Reveals Why She Clicked So Fast With Jacob Elordi
Margot Robbie Reveals Why She Clicked So Fast With Jacob Elordi -
 Piers Morgan Praised By Ukrainian President Over 'principled Stance' On Winter Olympics Controversy
Piers Morgan Praised By Ukrainian President Over 'principled Stance' On Winter Olympics Controversy -
 Halsey's Fiance Avan Jogia Shares Rare Update On Wedding Planning
Halsey's Fiance Avan Jogia Shares Rare Update On Wedding Planning -
 Instagram Head Adam Mosseri Says Users Cannot Be Clinically Addicted To App
Instagram Head Adam Mosseri Says Users Cannot Be Clinically Addicted To App -
 James Van Der Beek Was Working On THIS Secret Project Before Death
James Van Der Beek Was Working On THIS Secret Project Before Death -
 Las Vegas Father Shoots Daughter's Boyfriend, Then Calls Police Himself
Las Vegas Father Shoots Daughter's Boyfriend, Then Calls Police Himself -
 'Hunger Games' Star Jena Malone Shocks Fans With Huge Announcement
'Hunger Games' Star Jena Malone Shocks Fans With Huge Announcement -
 Ex-OpenAI Researcher Quits Over ChatGPT Ads
Ex-OpenAI Researcher Quits Over ChatGPT Ads