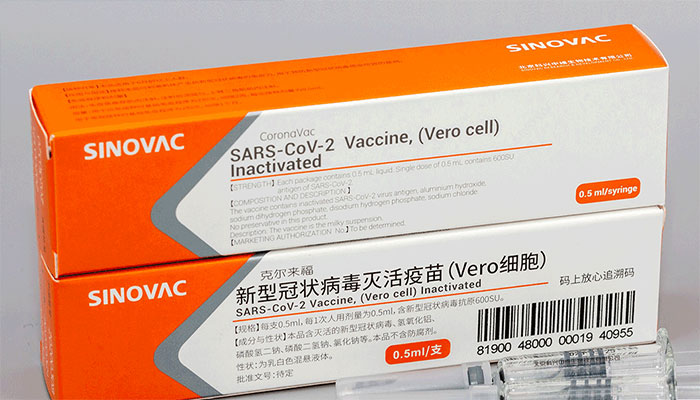
DRAP allows emergency authorisation to fifth Covid-19 vaccine
The registration board of Drug Regulatory Authority of Pakistan (DRAP) has allowed another Chinese vaccine, CoronaVac, for COVID-19, officials said on Thursday
KARACHI: The registration board of Drug Regulatory Authority of Pakistan (DRAP) has allowed another Chinese vaccine, CoronaVac, for COVID-19, officials said on Thursday adding that despite its low efficacy shown during the trials, the vaccine was allowed to counter the third wave of pandemic in Pakistan.
“DRAP’s registration board in its 304th meeting on Thursday gave Emergency Use Authorization (EUA) to CoronaVac vaccine, developed by the Sinovac Life Sciences Company Ltd., based in Beijing, China," an official of the Drug Regulatory Authority told The News on Thursday. This would be the fifth Covid-19 vaccine and the third Chinese vaccine to be allowed to be used in Pakistan as earlier, Pakistan had granted Emergency Use Authorization (EUA) to China’s two dose Sinopharm and single dose Convidecia vaccine developed by the Cansino Biologicals Inc. Oxford's AstraZeneca’s Covishield and Russian Sputnik V vaccine have also been given Emergency Use Authorization in Pakistan. Two Chinese and one Russian vaccines are being used in the public and private sectors, officials said.
The DRAP official further said an expert committee had recommended emergency use authorization for CoronaVac vaccine, although its data was not published and overall efficacy was around 56 percent. “But in Turkey and Indonesia, efficacy of CoronaVac has been found to be 91.25% and 85.3% respectively. Besides, it is already being used in China, Turkey, Indonesia, Brazil and some Middle Eastern countries.
The company provided its safety and efficacy data to the expert committee and on its recommendations, the DRAP’s registration board allowed the vaccine as we desperately need vaccines in Pakistan,” the DRAP official added.
The CoronaVac is a traditional vaccine, which can be stored at 2-8 degrees Celsius, which makes it ideal for countries like Pakistan where they don’t have ultra cold chain refrigerators. It is worth mentioning here that phase III clinical trials of a triple-dose Chinese vaccine, developed by the Anhui Zhifei Longcom Biopharmaceutical Company Ltd, are also underway in Lahore, Faisalabad, and other cities of Pakistan.
-
 Prince William Should Focus On 'family Business' After Andrew Blunder
Prince William Should Focus On 'family Business' After Andrew Blunder -
 Katherine Schwarzenegger Pratt 'brought To Tears' By Sister-in-law's Gesture
Katherine Schwarzenegger Pratt 'brought To Tears' By Sister-in-law's Gesture -
 Prince William Makes Bold Claim About Britain's Creative Industry At BAFTA
Prince William Makes Bold Claim About Britain's Creative Industry At BAFTA -
 Andrew Mountbatten Windsor Insulting 'catchphrase' That Degarded Staff
Andrew Mountbatten Windsor Insulting 'catchphrase' That Degarded Staff -
 Kate Middleton, Princess Beatrice 'undercurrent Tension' Comes To Surface
Kate Middleton, Princess Beatrice 'undercurrent Tension' Comes To Surface -
 'Grey's Anatomy' Alum Katherine Heigl Reveals Why She Stayed Silent After Eric Dane Loss
'Grey's Anatomy' Alum Katherine Heigl Reveals Why She Stayed Silent After Eric Dane Loss -
 Host Alan Cumming Thanks BAFTAs Audience For Understanding After Tourette’s Interruption From Activist
Host Alan Cumming Thanks BAFTAs Audience For Understanding After Tourette’s Interruption From Activist -
 Jennifer Garner Reveals Why Reunion With Judy Greer Makes Fans 'lose Their Minds'
Jennifer Garner Reveals Why Reunion With Judy Greer Makes Fans 'lose Their Minds' -
 Chris Hemsworth Makes Shocking Confession About His Kids' Reaction To His Fame
Chris Hemsworth Makes Shocking Confession About His Kids' Reaction To His Fame -
 Wiz Khalifa Reveals Unconventional Birthday Punch Tradition With Teenage Son In New Video
Wiz Khalifa Reveals Unconventional Birthday Punch Tradition With Teenage Son In New Video -
 BAFTAs 2026: Kerry Washington Makes Debut In Custom Prada Gown
BAFTAs 2026: Kerry Washington Makes Debut In Custom Prada Gown -
 Jennifer Lopez Gets Emotional As Twins Max And Emme Turn 18
Jennifer Lopez Gets Emotional As Twins Max And Emme Turn 18 -
 Andrew Mountbatten Windsor Blunders Are Result Of 'conspiracy Of Silence'
Andrew Mountbatten Windsor Blunders Are Result Of 'conspiracy Of Silence' -
 Keith Urban Fires Entire Management Team After Divorcing Nicole Kidman
Keith Urban Fires Entire Management Team After Divorcing Nicole Kidman -
 Kylie Jenner Marks Death Anniversary Of Hairstylist Jesus Guerrero With '222' Tribute
Kylie Jenner Marks Death Anniversary Of Hairstylist Jesus Guerrero With '222' Tribute -
 Daniel Radcliffe On How It's Like Seeing New Harry Potter Cast Years Later
Daniel Radcliffe On How It's Like Seeing New Harry Potter Cast Years Later