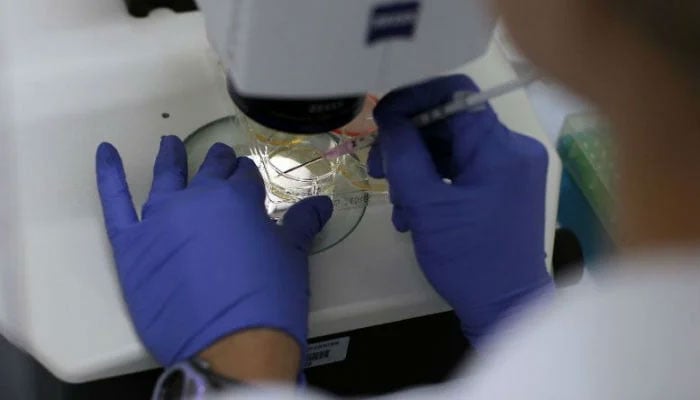
Concern voiced over illegal practice of stem cell therapy
ISLAMABAD: Pakistan Blood and Marrow Transplant (PBMT) Group has expressed serious concerns over illegal practice of stem cell therapy and regenerative medicine centers administering non-FDA, non-NBC approved commercial stem cell products.
They urge the regulatory bodies to take appropriate action to stop this corrupt practice. Pakistan Blood and Marrow Transplant (PBMT) Group includes cellular therapy and stem cell transplant experts from all over Pakistan with decades of National and International experience.
President of PBMT, Prof. Parvez Ahmed, has told The News that over the last few years, different centers in large cities of Pakistan are offering stem cell therapy for different diseases like hair fall, dementia, muscular dystrophies, infertility, autism and numerous other disorders.
These centers portray stem cell therapy as last hope and only chance of cure, luring patients to receive this extremely expensive experimental treatment which is neither approved in Pakistan nor elsewhere in the world. This raises serious safety, ethical, scientific and legal concerns.
The centers offering stem cell therapy are offering non-FDA approved treatment without regulatory approval in Pakistan. The treatment offered doesn’t have a scientific proof of their efficacy and can cause serious side effects like bacterial infection, hepatitis, HIV, potential for cancer.
The non-FDA approved stem cell treatment can only be offered as a part of clinical trial duly approved by NBC and its current use amounts to illegal and corrupt practice.
Cellular therapies including stem cell therapy is a highly specialized treatment modality that is mostly experimental. Worldwide more than 1000 clinical trials are currently being carried out to see the clinical efficacy of stem cell therapy. Most of the previously conducted trials were negative trials resulting in failure to achieve regulatory approvals.
World’s largest drug regulatory body, US FDA has stated that currently, the only stem cell treatments approved by the Food and Drug Administration (FDA) are products that treat certain cancers and disorders of the blood and immune system (CAR-T cell therapy and gene therapy).
If the products are being used for arthritis, injury-related pain, chronic joint pain, anti-aging or other health issues, they have not been approved by FDA and are being marketed illegally’.
In Pakistan, National bio-ethical committee (NBC) and human organ transplant authority (HOTA) are regulatory authorities to safeguard the rights, safety and wellbeing of patients. Their statement on use of stem cell therapy states that all centers performing stem cell research and therapy should be registered with the HOTA for accreditation, on the basis of their technical competence (in stem cell collection procedures, enumeration, cryopreservation, stem cell viability studies) and ethical review and oversight procedures. All proposals involving stem cells of any source for research or non-approved therapy should be cleared by Human Organ Transplant Authority (HOTA) Sub-Committee, through the National Bioethics Committee.
-
 Japan: PM Takaichi Flags China ‘Coercion,’ Pledges Defence Security Overhaul
Japan: PM Takaichi Flags China ‘Coercion,’ Pledges Defence Security Overhaul -
 Angorie Rice Spills The Beans On Major Details From Season 2 Of ' The Last Thing He Told Me'
Angorie Rice Spills The Beans On Major Details From Season 2 Of ' The Last Thing He Told Me' -
 Questions Raised Over Andrew Mountbatten-Windsor's Line Of Succession
Questions Raised Over Andrew Mountbatten-Windsor's Line Of Succession -
 'Shameless' Sarah Ferguson 'pressuring' Princess Eugenie, Beatrice For Major Reason
'Shameless' Sarah Ferguson 'pressuring' Princess Eugenie, Beatrice For Major Reason -
 Teacher Arrested After Confessing To Cocaine Use During Classes
Teacher Arrested After Confessing To Cocaine Use During Classes -
 Paul McCartney Talks 'very Emotional' Footage Of Late Wife Linda In New Doc
Paul McCartney Talks 'very Emotional' Footage Of Late Wife Linda In New Doc -
 Princess Beatrice, Princess Eugenie's Response To Andrew's Arrest Revealed
Princess Beatrice, Princess Eugenie's Response To Andrew's Arrest Revealed -
 King Charles And Princess Anne Bestow Honours At Windsor Castle
King Charles And Princess Anne Bestow Honours At Windsor Castle -
 King Charles 'worried' As Buckingham Palace, Royal Family Facing 'biggest Crisis'
King Charles 'worried' As Buckingham Palace, Royal Family Facing 'biggest Crisis' -
 Milo Ventimiglia Recalls First Meeting With Arielle Kebbel On The Sets Of 'Gilmore Girls' Amid New Project
Milo Ventimiglia Recalls First Meeting With Arielle Kebbel On The Sets Of 'Gilmore Girls' Amid New Project -
 Eric Dane Infuriated After ALS Diagnosis As He Feared The Disease Would Take Him Away From His Girls
Eric Dane Infuriated After ALS Diagnosis As He Feared The Disease Would Take Him Away From His Girls -
 It's A Boy! Luke Combs, Wife Nicole Welcome Third Child
It's A Boy! Luke Combs, Wife Nicole Welcome Third Child -
 Leading Astrophysicist Shot Dead At Southern California Home
Leading Astrophysicist Shot Dead At Southern California Home -
 Johnny Depp's Kind Gesture Towards Late 'Grey's Anatomy' Actor Eric Dane Before Death Laid Bare
Johnny Depp's Kind Gesture Towards Late 'Grey's Anatomy' Actor Eric Dane Before Death Laid Bare -
 How Princess Eugenie, Beatrice React To Andrew Arrest?
How Princess Eugenie, Beatrice React To Andrew Arrest? -
 Kylie Jenner 'convinced' Gwyneth Paltrow Is 'crushing' On Timothee Chalamet: 'It's Disrespectful'
Kylie Jenner 'convinced' Gwyneth Paltrow Is 'crushing' On Timothee Chalamet: 'It's Disrespectful'