New two-minute COVID-19 test approved for usage in US
The game-changing two-minute test has 'a 91% clinical specificity rate and a 99% clinical sensitivity rate'
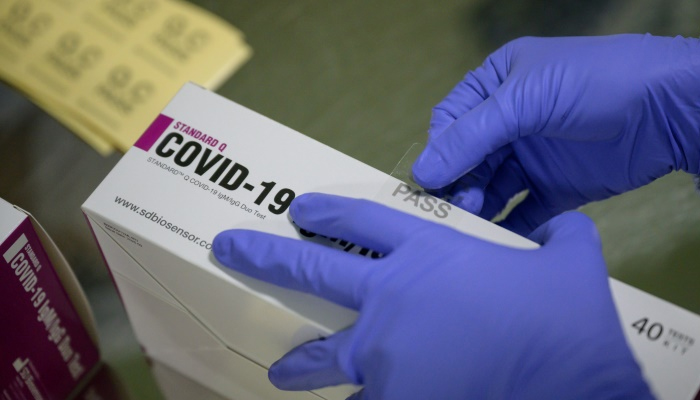
WASHINGTON: A new two-minute test to diagnose COVID-19 has been approved for usage by the US Food and Drug Administration (FDA), international media outlets reported on Tuesday.
According to Business Wire, the Los Angeles-based Bodysphere Inc announced that it was starting the distribution of its Two-Minute Testing Kit for diagnosis of the novel coronavirus after the FDA green-signaled it by way of an Emergency Use Authorization.
The game-changing two-minute test has "a 91% clinical specificity rate and a 99% clinical sensitivity rate" and delivers results "on site in as fast as two minutes", the publication added.
Reuters quoted Bodysphere as saying it was working with the Trump administration, as well as the state governments, to deliver the test — for emergency use — to the frontlines.
US President Donald Trump, who has praised the drug regulatory authority as "so great", had said March 19 he was pushing the FDA to approve similar tests and medicines as the COVID-19 panic surges. The US has so far recorded more than 3,000 deaths from the pandemic.
Last week, the body had approved Abbott Laboratories’ test that can deliver results within five minutes.
-
Jada Pinkett Smith details how her memoir combats 'shame' around alopecia
-
Billy Joel admits cancelling of tour due to brain disorder 'sounds a lot worse' than it is
-
Chester Bennington’s mental health story and lasting legacy
-
Yerin Ha opens up about shocking diagnosis post ‘Bridgerton’ season 4
-
Everything to know about Justin Bieber's facial paralysis
-
Sarah Ferguson’s dual cancer journey
-
Demi Moore was left with ‘intense’ illness after ‘The Substance’
-
How Michael J. Fox helped Harrison Ford with his Parkinson's monologue