China approves trials for two more vaccines as world's scientists race to beat COVID-19 pandemic
China now has three different clinical trials for three possible coronavirus vaccines in the works
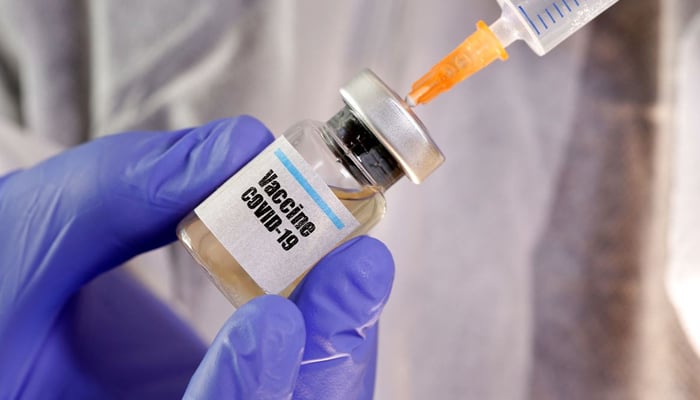
BEIJING: China has approved clinical trials for two more experimental vaccines to combat the novel coronavirus, officials said Tuesday, as the world's scientists race to beat the pandemic.
The vaccines use inactivated coronavirus pathogens, and the approvals pave the way for early-stage human trials, Wu Yuanbin, an official from China's Ministry of Science and Technology told a regular press briefing.
China's state food and drug administration on Monday approved one vaccine developed by a Beijing-based unit of Nasdaq-listed Sinovac Biotech, Wu said.
Another vaccine, being developed by the Wuhan Institute of Biological Products and the Wuhan Institute of Virology, was approved on Sunday, he added.
China now has three different clinical trials for three possible coronavirus vaccines in the works.
Beijing approved the first trial for a vaccine developed by the military-backed Academy of Military Medical Sciences and Hong Kong-listed biotech firm CanSino Bio on March 16.
That day the US drug developer Moderna said it had begun human tests for their vaccine with the US National Institutes of Health.
"Vaccination of subjects during the first phase of clinical trials and the recruitment of volunteers for the second phase of clinical trials began on April 9," Wu said.
"It's the world's first novel coronavirus vaccine to initiate Phase II clinical studies."
There are currently no approved vaccines or medication for the COVID-19 disease, which has killed more than 120,000 people worldwide and infected nearly two million.
Chinese teams were also racing to develop vaccines using other methods including using attenuated influenza virus vectors or injecting specific nucleic acid.
Several of these projects are currently undergoing animal testing and quality inspections, Wu said.
"The vaccines using the above technical methods are expected to be submitted for clinical trials in April and May," he added.
Experts have raised hopes that a vaccine could be ready within 18 months.
-
Sterling K. Brown's wife Ryan Michelle Bathe reveals initial hesitation before taking on new role
-
Rising energy costs put UK manufacturing competitiveness at risk, industry groups warn
-
Liza Minnelli recalls rare backstage memory with mum Judy Garland in new memoir
-
Armed intruder shot dead at Trump's Mar-a-Lago residence: US Secret Service
-
Kurt Russell spills the beans on his plans for milestone birthday this year: 'Looking forward to it'
-
11-year-old allegedly kills father over confiscated Nintendo Switch
-
Police officer arrested over alleged assault hours after oath-taking
-
Maxwell seeks to block further release of Epstein files, calls law ‘unconstitutional’